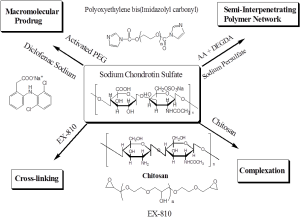近五年之研究計畫內容與主要研究成果:
Drug Delivery Systems:
We have devoted ourselves to developing novel polymers for drug/gene delivery systems based on chitosan (ChI) and/or chondroitin sulfate (CS), which are natural, biocompatible, biodegradable, and nontoxic polysaccharides. Because of highly water solubility of CS, it is impossible to use nascent CS as a drug delivery carrier. We have used physical and/or chemical approaches to modify CS as a good carrier for therapeutic agents. Our research fields are simply organized into four categories as the scheme shown below. All approaches are dedicated to sustained and/or controlled drug delivery.
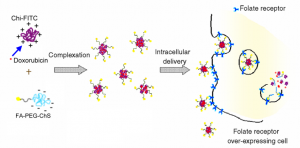
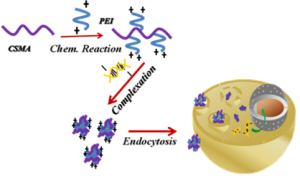
The low molecular weight of folic acid (FA, Fw=441.4 g/mol, vitamin B9) binds selectively to folate receptor (FR), a glycosylphosphaidylinositol-anchored cell surface receptor overexpressed in many solid tumors. These nutrient pathways are attractive since they are directly related to cell proliferation. The most aggressive tumor cells will cause an increase in cellular uptake in the presence of particles with the FA moiety. Using FRs as a target for molecular imaging or drug delivery systems has been commonly realized. We synthesized the folate–mediated CS (FA-CS) for tumor targeting by adopting the synthesis skill from the macromolecular prodrugs stated in the upper left corner. We have encapsulated an anticancer agent, doxorubicin (DOX), during the formation of complexes with ChI. Polyelectrolyte complexation (PEC) grafted with a fluorescent dye (FITC) served as a platform for online imaging cellular internalization as a strategy shown in Figure 1. The outcome result was accepted for publication in J. Biomater. Sci. (2011). We also did the succinalation of ChI for gene delivery systems. The succinated ChI (ChI-succ) was prepared to solve the solubility problem of nascent ChI under the physiological condition. The complexation between ChI-succ and plasmid DNA was published as an article in Nanomedicne: NBM, 2011.
Figure 1. The strategy of preparing complex nanoparticles for folate-mediated endocytosis
We introduced the double bonds to a hydrophobic polycaprolactone (PCL). The modified PCL was grafted onto methacrylated CS (CSMA) through a radical reaction. By controlling the composition between CSMA and PCL, the various morphologies of nanoparticles were obtainable. The synthesis and physicochemical characterization of CSMA with 70% methacrylation and PCL with the molecular weight 2000 g/mol was published in Biomacromolecues (2008). The FA moiety was further grafted onto CSMA-g-PCL to improve cellular uptake in FR overexpressed tumor cells. The potential of delivery of DOX to 6 lung cancer cells were tested and the outcome result was published as an article in Int. J. Nanomed. (2012, Figure 2). The PCL composition of CSMA-g-PCL on morphology was published in J. Biomater. Sci. (2012, Figure 3). In the parallel study, the graft copolymerization of CSMA and Pluronic F127 (PF127) with the FA moiety was prepared for drug delivery systems. The nanoparticles containing FA consistently showed the better cellular uptake in FR-overexpressed tumor cells (EJPS, 2009). Because of low yield in preparation of CSMA-g-PCL using the radical graft method, an alternative way to synthesize CSMA-g-PCL was done by atom transfer radical addition (ATRA). An anticancer drug, camptothecin (CPT), was loaded to kill the non-small-cell lung cancer CRL-5802 cells, overexpressing CD44 receptors. The outcome result was submitted for publication as an article in Mol. Pharm. (2014). Chemical conjugation of DOX as well as FA with CSMA-g-PCL for enhanced intracellular drug delivery was conducted and accepted for publication as an article in RSC. Adv (2014).
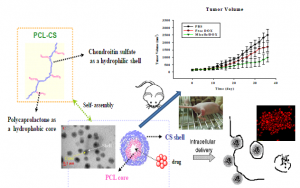
Figure 2. The antitumor activity of doxorubicin (DOX)-loaded CSMA-g-PCL (Micelle DOX) against NCI-H358 human non-small-cell lung cancers.
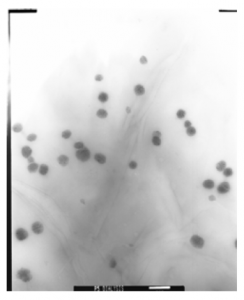
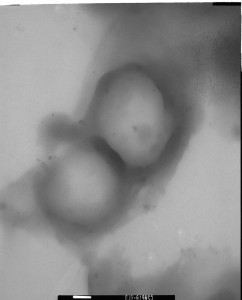
Figure 3. TEM images of (A) the weight percent of hydrophilic larger than hydrophobic segments formed spherical micelles, (B) the hydrophilic similar to hydrophobic segments expected to form rod-like, and (C) the hydrophilic less than hydrophobic segments expected to form polymersomes. (1 bar = 500 nm)
Dual-Diagnosis Systems:
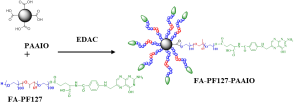
We developed novel multifunctional magnetic nanoparticles (MNP) with the FA moiety. Instead of dispersing iron oxide particles (Fe3O4) in micelles commonly reported in literature, the chemical conjugation of the iron oxide particles and PF127 was prepared. The FA-modified PF127-PAAIO was used for MRI diagnosis as well as for chemical cancer therapy. The result has been published in Biomaterials (2009, Figure 4).
Figure 4. Schematic illustration of chemical formation of FA-PF127-PAAIO
Similarly, the exposed free carboxylic group of PAAIO surface was used for covalent attachment of a fluorescent dye, Rhodamine 123 (Rh123) to yield PAAIO-Rh123, which permits applications in fluorescence imaging. PAAIO-Rh123 is therefore a dual-modality molecular probe. In order to endow specific properties to compounds that target cancer cells and prevent recognition by the reticuloendothelial system (RES), FA-linked poly(ethylene glycol) (FA-PEG) was further conjugated onto PAAIO-Rh123. The result was published in Biomaterials (2010). Currently, a blood-brain-barrier (BBB)-penetrating peptide, Angiopep was further linked onto the surface of PF127-PAAIO for brain targeting delivery, which has been accepted for publication as an article in J. Mater. Chem. B (2014). The comparison of chemically and physically coated iron oxide nanoparticles with poly(ethylene glycol)-b-poly(ε-caprolactone)-g-poly(acrylic acid) for imaging and chemotherapy was submitted for publication in J. Biomed. Nanotech. (in press, doi:10.1166/jbn.2014.2012).
Gene Delivery Systems:
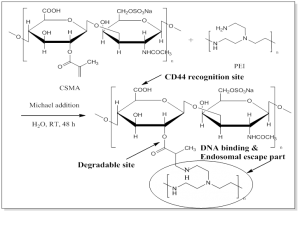
Combining CSMA with a cationic polymer to reduce the intrinsic cytotoxicity of the cationic polymer itself is our current interest. We separately grafted PEI and poly(dimethylaminoethyl methacrylate (PDMAEMA) onto CSMA and evaluated their potential for gene delivery systems. The promising results were accepted for publication in Molecular Pharmaceutics (CSMA-PEI, 2013, Figure 5) and Advanced Healthcare Materials (CSMA-PDMAEMA, 2013). The further introducing FA to CSMA-PEI or CSMA-PDMAEMA for tumor targeting has been accepted for publication as an article in J. Biomed. Nanotech. (doi:10.1166/jbn.2015.2081). In addition, PEI-graft-chitosan as a gene carrier for glial cell line-derived neurotrophic factor gene delivery system collaborated with National Tsing Hua University was published as an article in Int. J. Nanomed. (2014) as well.
Figure 5. Schematic illustration of CSMA-PEI as a nonviral gene vector
Diagnosis and/or Gene Delivery Systems:
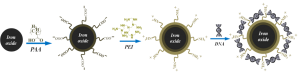
The electrostatic interactions of PEI and PAAIO endow themselves to be used as a magnetoplex for gene delivery. The major advantage of using magnetofection is to save transfection time and improve transgene expression (Curr. Pharm. Design 2011 and Langmuir 2012, Figure 6). Using PEI-PAAIO as a gene transfection carrier, cytotoxicity was also an issue at a high dose. Currently, we are testing if CSMA-PEI-coated PAAIO will be a better gene carrier than CSMA-PEI or PEI-PAAIO for microRNA delivery. The promising result is written up as an article for publication in near future.
Figure 6. Preparation of PEI-PAAIO/pDNA polyplexes for gene delivery systems
Besides, PDMAEMA was directly grafted from the surface of Fe3O4 using one pot reaction via atom transfer radical polymerization (ATRP). We demonstrated this PDMAEMA-decorated Fe3O4 could be used as a gene carrier as well as a MRI contrast agent. The result was published as an article in Journal Materials Chemistry B (2013).
Drug/Gene Co-Delivery Systems:
Pluronic® polymers were copolymerized with PDMAEMA with small amounts of poly(acrylic acid) (PAA). The COOH groups of PAA were used to link molecular ligands for targeting and fluorescent dyes for imaging applications. The outcome result was published in Int. J. Nanomed. (2013). In addition, three Pluronic® polymers with different hydrophilic/lipophilic balance (HLB) values were designed for co-delivering pDNA and an anticancer drug, epirubicin was published as an article in RSC Adv. (2014). The efficiency of using linear and star-shaped copolymers as pDNA/doxorubicin co-delivery carriers was compared, where PCL was used as a hydrophobic core and PDMAEMA as a cationic moiety to condense pDNA (RSC Adv, 2014).