一、研究新知
-
螢光奈米鑽石標記:提供幹細胞追蹤與單一細胞影像分析的新方向
(中央研究院 張煥正教授提供)
摘自Nature Nanotechnology(2013)doi:10.1038/nnano.2013.147Received
04 October 2012 Accepted 26 June 2013 Published online 04 August 2013幹細胞具有維持組織的生理恆定並修復組織內細胞損傷的功能。因此如何確切掌控幹細胞移植進入生物體後的分布動向,成為幹細胞治療應用成功與否的關鍵。中央研究院細胞與個體生物學研究所特聘講座游正博博士與原子與分子科學研究所特聘研究員張煥正博士所領導的研究團隊最近發表於國際高影響指數的學術期刊「自然奈米科技」(Nature Nanotechnology),提出結合數種新穎技術,建構一個細胞追蹤方法,能偵測移植到肺部組織幹細胞的分布,並取得高解析單一細胞的影像。這個方法可以應用於未來觀測與評估幹細胞移植後在體內修復受損組織的能力,與探討影響幹細胞移植相關的因子與機制。
此研究係結合本院跨領域的學者之專長而完成。首先,細胞與個體生物學研究所游正博博士所領導的幹細胞研究團隊,利用醣蛋白質體學的研究策略,針對不同類別的肺部細胞所表現的醣蛋白作有系統分析,具體的找出肺部幹細胞具專一性的細胞表面醣蛋白。以流式細胞儀從新生小鼠肺部組織中分離出高純度的肺部幹細胞,發現具有自體增生及可誘導分化為第二型肺泡細胞再分化成第一型肺泡細胞的幹細胞特性。接下來,原子與分子科學研究所張煥正博士研究團隊,透過獨創的「螢光奈米鑽石」將肺部幹細胞進行螢光影像標記。由碳元素所構成螢光奈米鑽石具有高度的化學與光學穩定性及生物相容性,而鑑定標記後的肺部幹細胞的存活度、細胞的生長情況、及細胞分化能力等幹細胞特性,並不受螢光奈米鑽石標記的影響。
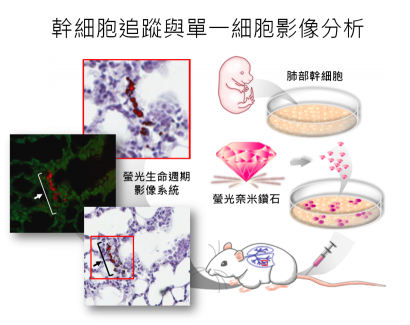
研究團隊們進一步將螢光奈米鑽石標記後的肺部幹細胞以靜脈注射移植小鼠體內,利用流式細胞儀分析不同組織內的肺部幹細胞所分布情形,發現只有肺部組織有偵測到標記肺部幹細胞訊號。另一方面,利用螢光奈米鑽石的螢光生命週期遠大於組織內自體螢光的特性,藉由自行改良之共軛焦顯微鏡的「螢光生命週期影像系統」可區分出標記後的肺部幹細胞螢光與組織本身自體螢光,確定所移植的肺部幹細胞位於肺部組織,並且位於支氣管與肺泡交界處,因此可應用於體內細胞追蹤並可得到單一細胞超高解析度的顯影。
螢光標示分子的各種巧妙應用,立下了分子生物學的里程碑。隨之而起的需要是更亮、更持久、生物相容性更好的標示記號。本研究所利用的螢光奈米鑽石除了具有以上優點外,更可以利用生命週期螢光顯微鏡影像技術區分出螢光生命週期較長的螢光奈米鑽石,並加以追蹤在組織的分布情形。研究團隊將肺部已損傷的小鼠移植螢光奈米鑽石標定的肺部幹細胞後,實驗結果發現以螢光奈米鑽石標定的肺部幹細胞不僅會回到受傷的肺部區域來進行修補並再生出新的肺部組織,也能夠藉由共軛焦顯微鏡建構螢光生命週期影像系統來追蹤體內微量的肺部幹細胞。此項成果可以為奈米科學和生物醫學的應用開啟一個新方向,盼望在未來疾病或癌症幹細胞的治療領域,可繼續發展出可能的臨床應用。Stem cell therapy has the potential to repair and regenerate damaged tissues. Unlocking this potential requires effective methods both for isolating and expanding stem cells and for tracking their ultimate fate when introduced into their host. A study has been published online in the high impact journal Nature Nanotechnology describes new methods for isolating, expanding, and labeling lung stem cells, that uses fluorescent nanodiamonds to track the fate of individual cells. This technology may offer insights into the factors that determine the acceptance of transplanted stem cells and their ability to regenerate within a host.
The labs, headed by John Yu, M.D., Ph.D., Distinguished Chair of Institute of Cellular & Organismic Biology, and Huan-Cheng Chang, Ph.D., Distinguished Research Fellow, Institute of Atomic and Molecular Sciences, Academia Sinica, collaborated on the work.First, they developed a new stem cell isolation method for lung stem cells (LSCs), using cell surface glycoprotein markers discovered by specialized proteomic analysis. They demonstrate that cells isolated with this method possess not only the abilities to self-renew and differentiate into lung tissues, but also the in vivo homing and regenerative abilities expected of a LSC. Next, they used fluorescent nanodiamonds (FNDs) to label these cells. FNDs are novel imaging probes which are not only chemically robust and fluorescently stable, but also biologically inert and nontoxic. These nanoparticles can be readily taken up by cells through endocytosis. When excited by green-yellow light, the built-in fluorophores emit stable far-red emission with a fluorescence lifetime of up to 15 ns, much longer than 1 ~ 4 ns of endogenous and exogenous fluorophores commonly used in cell biology. This property makes it possible to separate FND emission from strong autofluorescence backgrounds of host tissue, using special strategies described in this manuscript.
The authors successfully demonstrate the use of this system to isolate LSCs, engraft them in mouse models of lung injury, and track single cell fates. They illustrate that FND-labeled LSCs preferentially home to injured lungs more rapidly than to uninjured controls, enabling the lung epithelium to be restored more rapidly. In addition to demonstrating the therapeutic potential of such treatment, these results also support an “active homing” model in which transplanted LSCs proactively migrate to injured tissue, as opposed to non-specific/passive entrapment.
Yu and Chang suggest that this method has broad applicability to stem cell research, and that similar techniques could be used to monitor the uptake of different kinds of stem cells in other tissues.
The full article entitled “Tracking the engraftment and regenerative capabilities of transplanted lung stem cells using fluorescent nanodiamonds” is available at the Nature Nanotechnology website
