My research contributed to two topics: the Development of a solid-phase synthetic method for peptides and macromolecules and the Applications of dendrimers.
I.Development of solid-phase synthesis for functional peptides and dendrimers
A.Peptides synthesis
1. A new solid-phase synthetic method
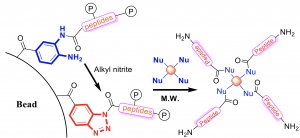
The preparation of C-terminal functionalized peptide is one difficulty in solid-phase peptide synthesis (SPPS) because the preparation is from C-terminal to N-terminal. Several methods were reported, but they either required complicated purifications or low yield. We developed a convenient and efficient chemical toolbox for the on-resin C-terminal functionalization of peptides for this issue. By transforming resin-bound 3,4-diaminobenzoic acid (Dbz) species with isoamyl nitrite, the resulting resin-bound benzotriazole (Bt) entity can be efficiently displaced by nucleophiles during the cleavage step in a short reaction time.1 This method allowed the introduction of polymers and small molecules as nucleophiles. (Figure 1) 
Figure 1. Schematic presentation of the preparation of C-terminal functionalized peptide through Dbz/Bt resin
2.Branch peptides and amphiphilic analogs
Branched peptides are a particular category of peptides with branched structures and could only be prepared from chemical synthesis because of no natural version of such peptides. Although SPDS is a popular approach, the classical staining method is challenging to determine the progress of the reaction and usually leads to defects. Recently, amphiphilic analogs have been draining attention due to their potential to assemble as mega molecules for various purposes. However, their preparation is more complex, especially for biomedical applications.
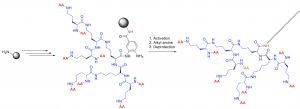
By the Dbz/Bt resin, linear peptides could be synthesized through classical SPPS. After reaching the desired length, the activation of Dbz to Bt allowed the introduction of lysine to give branched peptides in one step. By this approach, four branched peptides could be prepared without chromatographic purification. (Figure 2a )2
Through a similar approach, amphiphilic branched peptides were prepared. By synthesizing polylysine and installing peripheral groups, the activation of Dbz to Bt was used to introduce the aliphatic chain. Because the unreacted polysine remains on resin, a pure product could be collected with simple precipitation. (Figure 2b; in progress)
a.)
b.)
Figure 2. Preparation of a.) Branched peptides (route A), amino alcohol (Route B), and b.) amphiphilic branched peptides.
B. Solid-phase dendrimer synthesis
Dendrimers are a specific category of polymers and generate characteristic behavior due to the dense distribution of peripheral groups and internal cavities. Regardless of their potential biomedical applications, their preparation to reach biomedical requirements is a concern. Moreover, no reported GMP-grade production also damages its values in clinical applications. Using solid-phase synthesis, the preparation of the dendrimer reached the requirement for the first time. Meanwhile, the short time frame also improves their Development.
a.)
b.)
c.)
Figure 4. Preparation of a.)inverse PAMAM dendrimers and b.)triazine dendrimers. c) defect lysine dendrimer
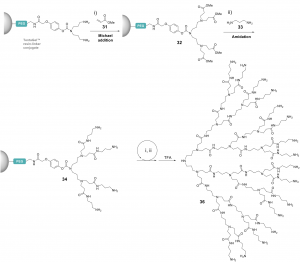
A concise synthesis of inverse poly(amidoamine) dendrons was developed. Each dendron generation was constructed via a single reaction upon the introduction of AB2-type of monomers. G2 to G5 dendrons were constructed according to this method with 93%~78% yields within five days.3 By reducing the loading rate of using resin, this method was applied to prepare G7 analogs, the largest dendrimer produced by solid-phase dendrimer synthesis (SPDS) on record.4 (Figure 4a)
A similar method was applied to prepare triazine dendrimers. Besides, a pair of orthogonal staining methods involving ninhydrin and Alizarin R was used to monitor the presence of alkyl amines or aryl chlorides, respectively. This staining method was applied to the SPDS of a small library of triazine dendrimers. Meanwhile, peripheral dichlorotriazines could be reacted selectively with two different amine nucleophiles to yield a multifunctional surface in high yield. The whole preparation process could be accomplished within a week.5 (Figure 4b)
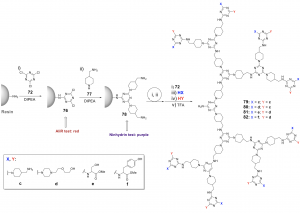
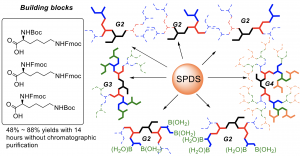
Through the concept of SPDS, an efficient solid-phase method has been reported to prepare well-defined lysine defect dendrimers. (Figure 4c) Pure G2 to G4 lysine defect dendrimers were prepared with 48 – 95% yields within 13 hours using orthogonally protected lysine residues. Remarkably, high-purity products were collected via precipitation without further purification steps.6
II Application of dendrimers and peptides.
1.Development of metallodendrimers catalysis for oxygen reduction
[CuY((G:2-6)-dendri-PAMAM-(Py)n)]2Y+ (Figure 6a) were prepared, and their ability to generate oxygen radical anions was investigated. The maximum catalytic efficiency was found to be 0.32 min–1‧M–1, and a positive dendritic effect was observed. The saturated kinetics revealed that the improved catalytic efficiency resulted from an enhanced binding affinity toward oxygen.7 To further investigate the possible cooperative effects of Cu ions, (G:2-6)-dendritic-PAMAM-(Py)n dendrimers were used to reduce oxygen in the presence of varying amounts of Cu ions. Spectrophotometric titration revealed the maximum numbers of active Cu ions in each dendrimer. Further analysis revealed that each Cu ion worked independently as an active center in the G2- to G4-dendrimer complexes. In contrast, active bimetallic species were present in the G5- and G6-dendrimer complexes. These findings suggest that the catalytic behavior of metallodendrimers can be manipulated by adjusting the size of the dendrimer–metal complex. For the investigation of the size effect in the cleavage of DNA plasmid, the [CuY((G:2-6)-dendritic-PAMAM-(Py)n)]2Y+ (Y=3, or 6) were prepared and subjected to DNA cleavage experiment. The result indicates that the increasing molecule size can also improve their activity without altering the number of Cu ions.8 A computer-aided EPR study of these complexes identifies different complexes by increasing the Cu(II)/Py molar ratio for the different generations. (Figure 6b) Binuclear EPR-silent complexes were formed at the highest generations. The complexed Cu(II) ions were reduced, starting from the most exposed at the surface. The spin trap for capturing hydroxyl radicals clearly observed the EPR become visible after all Cu(II) ions were reduced to Cu(I).9
a.)
b.)
(c)
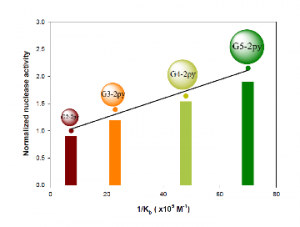
Figure 6. a.) representative structure of bi-pyridine modified PAMAM dendrimers, b.) Example of PBN-trapped transient radical for G2 dendrimer with Cu(II) and DTT concentration of 0.03 M. c.) Correlation of binding strength of Cu complexes of PAMAM dendrimers with DNA plasmid and their nuclease activity.
The Kb values of the G2 to G6 apodendrimers for DNA plasmid were 7.4, 23, 48, 70, and 280 µM−1, respectively. The chemical nuclease activities of the corresponding complexes were determined, and an apparent positive dendritic effect was observed. Further analysis indicated a linear correlation between the Kb values of the G2 to G5 apodendrimers and the nuclease activity of the corresponding complexes. (Figure 6c) This observation indicated the importance of substrate binding affinity for macromolecular nuclease activity. In addition, performed inhibition studies indicated that the hydroxyl radical was the active species responsible for the plasmid cleavage.10
2. Synthesis of boronic acid-decorated dendrimers as a selective glucose sensor
Boronic acids are widely in various fields, including polymer chemistry. However, it is rare to use in dendrimers. During our study, we found the most challenging task is the preparation of boronic acid-decorated dendrimer in consistent quality, especially for a high ratio of functionalization. The primary reason is that the presence of amines in proximity caused the loss of boronic acid instead of hydroxyl groups.11
The resulting functionalized dendrimers were subjected to carbohydrate binding experiments. The result indicated higher generation of functionalized dendrimers demonstrated selective and high-affinity binding to glucose over other monosaccharides. Such selectivity usually requires bi-boronic acid moieties in one molecule with well-designed molecular architecture.11 (Figure 7)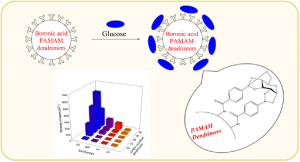
Figure 7. Boronic acid-decorated PAMAM dendrimer binds glucose and the binding affinity with various carbohydrates.
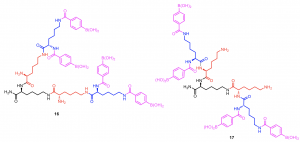
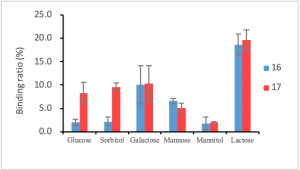
The SPDS method was applied to prepare a pair of 4-carboxyphenylboronic acid-decorated defect dendrimers with the same number of boronic acids. The binding affinity of 16, in which the e-amines of G1 lysine are fractured, for glucose and sorbitol was four times that of 17. This investigation indicated the role of allocation and distribution of peripheries for dendrimer’s properties and activity.12
Figure 8. a.) Chemical structure of 16 and 17, b.) the binding affinity of 16 and 17 to various carbohydrates.
Reference
- Selvaraj, A.; Chen, H.-T.; Ya-Ting Huang, A.; Kao, C.-L., Chemical Science 2018, 9 (2), 345-349.
- Molakaseema, V.; Selvaraj, A.; Chen, H.-T.; Chen, Y.-W.; Liu, Y.-C.; Kao, C.-L., The Journal of Organic Chemistry 2022, 87 (1), 1-9.
- Huang, A. Y.-T.; Tsai, C.-H.; Chen, H.-Y.; Chen, H.-T.; Lu, C.-Y.; Lin, Y.-T.; Kao, C.-L., Chemical Communications 2013, 49 (51), 5784-5786.
- Kao, C.-L.; Huang, A. Y.-T.; Chen, H.-T., Macromolecular Rapid Communications 2017, 38 (12), 1700062.
- Huang, A. Y.-T.; Patra, S.; Chen, H.-T.; Kao, C.-L.; Simanek, E. E., Asian Journal of Organic Chemistry 2016, 5 (7), 860-864.
- Liao, Y.; Chan, Y.-T.; Molakaseema, V.; Selvaraj, A.; Chen, H.-T.; Wang, Y.-M.; Choo, Y.-M.; Kao, C.-L., ACS Omega 2022, 7 (26), 22896-22905.
- Kao, C.-L.; Tang, Y.-H.; Lin, Y. C.; Chiu, L.-T.; Chen, H.-T.; Hsu, S. C. N.; Hsieh, K.-C.; Lu, C.-Y.; Chen, Y.-L., Nanomedicine: Nanotechnology, Biology and Medicine 2011, 7 (3), 273-276.
- Tang, Y.-H.; Lin, Y. C.; Hsu, S. C. N.; Liou, S.-T.; Chen, H.-Y.; Hsieh, K.-C.; Chuang, W.-J.; Chiu, L.-T.; Chen, Y.-L.; Kao, C.-L., European Journal of Inorganic Chemistry 2015, 2015 (29), 4839-4847.
- Tang, Y.-H.; Cangiotti, M.; Kao, C.-L.; Ottaviani, M. F., The Journal of Physical Chemistry B 2017, 121 (46), 10498-10507.
- Tang, Y.-H.; Hsu, S. C. N.; Chen, P.-Y.; Liou, S.-t.; Chen, H.-T.; Wu, C. H.-Y.; Kao, C.-L., Pharmaceutics 2018, 10 (4), 258.
- Tsai, C.-H.; Tang, Y.-H.; Chen, H.-T.; Yao, Y.-W.; Chien, T.-C.; Kao, C.-L., Chemical Communications 2018, 54 (36), 4577-4580.
- Liao, Y.; Chan, Y.-T.; Molakaseema, V.; Selvaraj, A.; Chen, H.-T.; Wang, Y.-M.; Choo, Y.-M.; Kao, C.-L., ACS Omega 2022.